by Margaret Golz, Richard Wysk, Russell King. Colin Nolan-Cherry, & Stephen Bryant (North Carolina State University)
As presented at the 2018 Winter Simulation Conference
Abstract
Medical device companies have a growing interest in additive manufacturing (AM) for orthopedic implant production. AM provides an opportunity for economically feasible customized implants to supplement the current traditionally-manufactured, standard-sized prosthesis. In this paper, a simulation model is developed to analyze the supply chain of custom hip stem production using AM. A test case is implemented into the simulation model to determine the supply chain’s responses in key performance indices, such as production lead time of a uniquely customized prosthesis and resource utilization, to resource level variations based on current state of the practice for additive and subtractive manufacturing. The method of this research was to use a simulation focused on measuring the performance of the supply chain for hip stem implants in order to determine supply chain resource requirements.
AbsIntroduction and motivationtract
Arthroplasty, commonly called joint replacement, uses standard-sized prostheses that are pieced together to form a mechanical joint. Joint replacements become necessary when a patient suffers significant impairment from constant, severe joint pain and conservative treatment has been exhausted. Arthroplasty operations are relatively safe and can relieve pain as well as restore like-normal mobility. Due to significant stress and strain on the body through daily activities, implants will wear down and can fail; the 25-year survival rate of a hip implant is over 80% (Shan et al. 2014). When an implant fails, a much more costly, difficult, and riskier revision operation is necessary. Over a million total joint arthroplasty surgeries are performed annually in the U.S.; total hip arthroplasty (THA) comprise approximately a third of these (OrthoInfo 2017).
Figure 1 shows the three parts of a hip replacement implant: the femoral (hip) stem (inserted into the medullary cavity in the femur), acetabular component (replaces the socket in the pelvic region), and the femoral head (articulates the femoral stem to the acetabular component while allowing normal hip motion); each part comes in several sizes. Currently, traditional manufacturing methods are typically used to produce standard hip implant sizes and geometries for all patients. Conventional methods for manufacturing prosthesis hip stems depend on the type of material in use. Forging or investment casting are the typical procedures for medical device metals such as titanium, cobalt chromium, and stainless steel. After the basic geometry is shaped, rough machining, polishing, and coating complete the process (Zhang et al. 2009). Standard hip stems are not custom-made for individual patient use, therefore, not all prosthesis hip stems fit perfectly or are guaranteed to work well.
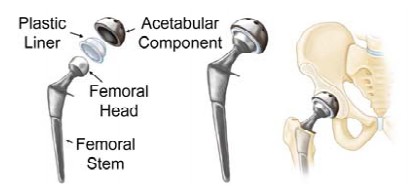
Figure 1: The images from left to right show the individual components of a total hip implant, a compiled hip implant, and the positioning of a hip implant into the joint (OrthoInfo 2017).
The medical field has grown increasingly interested in alternatives to standard medical implant sizes because of the anticipated benefits. Through customization, an implant can be adapted to accommodate the patient’s specific anatomy including bend stiffness (directly connected with aseptic loosening), leg length discrepancy, and highly deformed femurs (Cronskär et al. 2013). Aseptic loosening, the loosening of a prosthesis due to wear or failure of osseointegration, is a major cause of revision surgery. Custom hip implants often reduce the amount of bone removal necessary for aligning the hip stem during surgery. It is important to preserve as much bone as possible in case revision surgery is needed later. Surgery using custom hip implants should be relatively straightforward because the implant would better match the bone geometry of the patient, although it’s likely standard bone cuts would not be used. A simple fit also could lead to shorter surgery times and should reduce the likelihood of aseptic loosening (Harrysson et al. 2007). Research is in the early stages, but a small number of studies suggest customized prostheses have the potential to decrease the pain and suffering, risk of infection, and therapy time for the patient. It could possibly increase the life of the medical implant, postponing or eliminating revision surgery.
Additive manufacturing, also called rapid prototyping or 3D printing, has shown the capability to create custom implants from patient computer tomography (CT) scans; it has the ability to create unique, complex geometries relatively easily because it builds parts by laying down material a single cross section at a time. AM presents the opportunity to economically customize medical implants to better fit the patient’s anatomical geometry and activity level needs. A specific type of AM, Electron Beam Melting (EBM), is advantageous in the creation of medical devices because it can be used to produce prostheses using a titanium alloy that is already FDA-approved. Further, the surface finish of the titanium alloy Ti6Al4V when fabricated using EBM technology is biocompatible and has equivalent or possibly increased osseointegration compared to traditional implants (Haslauer et al. 2010; Thomsen et al. 2008). Parts built by EBM have less material waste relative to their standard part production by traditional manufacturing methods (Sing et al. 2015). EBM is currently in use for production of standard orthopedic implants (trabecular structures specifically) with CE-certification and FDA-approval (Arcam AB 2018).
There is a selected number of companies that are producing acetabular cups using Ti6Al4V with Arcam’s EBM machine. One such company, Adler Ortho Group, began producing CE-certified hip implants in 2007 (Arcam EBM 2018). The acetabular cups are only stock size and as of early 2014, over 90,000 implants have been produced by Adler Ortho and other companies; 40,000 of these EBM manufactured acetabular cups have been implanted. Also as of 2014, approximately 20 distinct medical devices fabricated using AM have gained FDA clearance for implantation (Gibson et al. 2015).
The continuous enhancement of AM has shown that it could potentially meet a portion of the current demand with custom implants or meet full market demand with a combination of standard and custom devices. For a medical device company, the AM technology opens the door to an unfamiliar mass production method; research is necessary to predict the required resources and limitations of large-scale production of custom implants. Modeling the supply chain can manifest upstream and downstream behaviors for the manufacturer. Supply chain simulation is a valuable tool that can provide insights of the supply and demand patterns as well as an evaluation of system resources. This research is focused on the development of a supply chain model of patient specific hip stems, model implementation into supply chain simulation software, and sensitivity analysis on resource levels.
Background
There have been multiple studies exploring how AM could be used for orthopedic medical implants. Two previous studies have used CAD modeling to design a custom femoral component of a knee replacement from a patient’s CT scan (Harrysson et al. 2007, Harrysson et al. 2003). In one study, finite element analysis (FEA) was performed on the custom and conventional CAD model files to evaluate stress distribution. The results showed several promising advantages of custom implants to the conventional ones: more even stress distribution decreasing the risk of premature loosening, decreased need for surgical interventions and filler components (due to a better fit), and about 40% less bone removal. The group determined that some of the disadvantages could be mitigated by the use of AM for component fabrication (Harrysson et al. 2007).
sadvantages could be mitigated by the use of AM for component fabrication (Harrysson et al. 2007). The second study created a custom femoral component of a canine knee implant using EBM and compared the creation time to traditional investment casting of the same custom part. The traditional method took more than three times as long as the EBM process to produce the custom implant. The component for the EBM machine was designed from a CT scan of the patient with contributions from orthopedic surgeons; file preparation for fabrication took 1.5 hours. The custom design was created out of Ti6Al4V powder, a material that has been FDA-approved for implantation into patients for particular medical devices created using AM. The group determined that EBM technology can be used to fabricate custom medical implants and design porous surfaces directly into the component (Harrysson et al. 2003).
Thomsen et al. (2008) performed an in vivo bone ingrowth analysis of Ti6Al4V EBM implants and found no appreciable difference in tissue response from polished, traditionally manufactured titanium alloy implants. The study also found osseointegration on the structural level. Prior to implantation, the parts were ultrasonically cleaned for 25 minutes. After washing, the implants were steam autoclaved in sterile bags.
hip stem prosthesis. The group fabricated 7 hip stem implants using an Arcam A2 EBM machine and machined the same set of implants for cost comparison. The implants were oriented vertically during file preparation in order to obtain the best surface finish. The study further simulated a build with 14 custom implants to observe the processing time for full capacity of the EBM machine. The simulation machine processing time for 7 and 14 implants was 36 hours and 38 hours respectively. The actual processing time for the 7 implant print approximately matched the simulation time; there was a cool-down time of 8 hours after the completion of the build. Wasted material from the build measured 24% of the total weight of the implants; the loss of material from the EBM-fabricated parts was 35% less than the conventional method. The study reported EBM file preparation (CAD file modification, support generation, and compilation) lasted about 40 minutes. Before printing, the EBM machine took just over an hour to warm up and draw the vacuum in the chamber. After printing, the operators had to remove excess powder and clean the machine/implants which also took approximately one hour. Following EBM printing, subtractive manufacturing with CNC machines contributed an additional 2 minutes per implant for milling and 1.5 hours per implant for grinding and polishing. The group concluded that AM custom implants are commercially viable and are economically advantageous over machined ones (Cronskär et al. 2013).
Research completed by Shouche (2016) presented a supply chain model for custom hip stems created with AM. Supply chain operations reference models were created for custom and traditional hip stems; the research focused on the comparison of the two models using performance metrics. The custom hip stem model was implemented using Arena simulation software and is shown in Figure 2; it was the inspiration for continued research on the topic. The model simulates the process of each patient’s order starting from the physician's diagnosis and finishing with the implant delivery to the hospital or discarding the implant if the part is insufficient. This process includes CAD modeling, EBM fabrication, and CNC machining to machine finish the implant. Resource requirements to satisfy an annual demand of 166,000 implants (approximately half of the THA performed annually) were determined through simulation; the system required 1,190 medical engineers to complete the CAD modeling and 110 EBM machines (both at 65% utilization). The study further determined that the metal powder to fabricate the hip stems and the number of EBM machines were the most sensitive to volume production change (Shouche 2016).
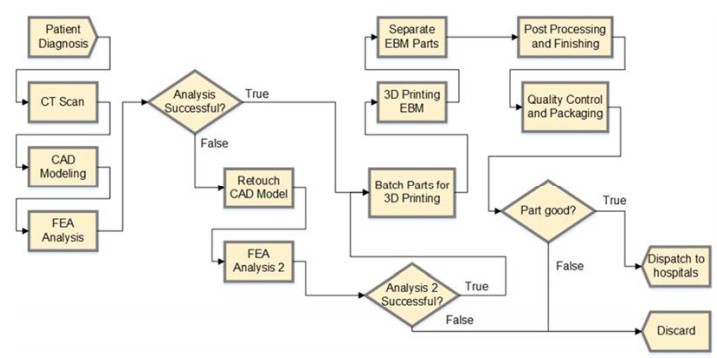
Figure 2: Supply chain model for customized hip stems implemented into Arena (Shouche 2016).
Methodology
The previous presented supply chain was the basis for further development of a more robust model for custom hip implants. This developed supply chain presents a more detailed model that breaks down general processes into more individualized pieces and includes implant transportation and delivery. This model ensures that every customer that enters the system completes the supply chain with a uniquely customized hip stem and can determine the sensitivity of the system to resource level variations. The parameter estimations are supported by literature. This research, unlike previous studies, focuses on the development and analysis of the supply chain and the required resources to benefit companies interested in market entry. The aim of this model is the addition of the above conditions and, in doing so, lacks aspects of the previous model presented by Shouche (2016). In particular, this developed model does not perform a financial analysis on the system and does not evaluate traditionally manufactured, standard-sized hip stems.
An anticipated supply chain was developed to predict material flow for customized hip stems from patient diagnosis to THA surgery. To evaluate the model, a test case was implemented into simulation software. As this is a predictive model, many assumptions had to be made about parameter values for the test case based on previous studies because existing values are not available. A set of key performance indices (KPIs) were used to assess the supply chain and necessary resources.
Supple Chain Model Development
The anticipated supply chain, shown in Figure 3, begins with CT scans after patient diagnosis for a custom hip prosthesis. CT scans are not typically performed for standard operations and are therefore considered as part of the custom hip stem supply chain. The medical engineer imports the provided CT scans into CAD modeling software and designs the patient-specific hip stem. The medical engineer then evaluates the design through Finite Element Analysis (FEA) to analyze the density map and identify any potential critical stresses or strains specific to that unique part. Of the evaluated virtual implants, those that pass inspection move onto the EBM operator to be added to a batch while those that fail require additional CAD work until they pass an FEA. The virtual hip stem implant file, after passing an FEA, goes to the EBM operator who aggregates multiple hip stem files to create a batch, orients the parts on a build platform, and adds additional support material needed for fabrication. Up until this point, the patient entity is represented virtually which nullifies the need for facility differentiation. The operator prepares the machine for printing (i.e. warms up the machine, draws the vacuum, etc.) and begins the build process. If a build fails, the entire batch must be re-printed. After the appropriate cooling time within the machine chamber, the EBM operator performs post-processing duties: extraction of the build plate, support material removal, and excess powder removal. The new custom hip stem is labeled and each part moves onto the finishing process individually. The remaining echelons of the supply chain could exist in one facility or in multiple facilities with transportation requirements between locations.

Figure 3: Anticipated supply chain of patient specific hip stems from the CT Scan of the patient to hospitalization (and implantation of the prostheses).
The CNC machinists operate CNC milling machines during the finishing process to complete milling and grinding/polishing. Milling smooths the cone end of the implant (the distal tip of device). The rougher middle portion of the implant where osseointegration is desired has sufficient surface finish from fabrication and does not require finishing. The proximal portion of the hip stem that connects to the femoral head component is manually grinded and polished. After the finishing process, if subsequent quality assurance (QA) inspections are met the implant is ultrasonically washed, sterilized in an autoclave, and packaged by a quality engineer. The implant is then distributed to the appropriate medical facility for surgical implementation. After the hip stem leaves the manufacturing facility, it is shipped directly to the hospital. As the AM hip stems are unique pieces, they will be shipped as individual packages, not in batches. The implant is held in the hospital until the patient undergoes surgery. Typically, medical implants are held as inventory by the manufacturer until the operation, but because the implants are personalized, the authors anticipate the medical facilities could hold the small inventory of custom hip stems for their patients prior to implantation.
The new supply chain strives to demonstrate how every custom hip stem order that enters the system is guaranteed to exit the system with success, an important difference from previous work.
Simulation Implementation: Controls and Parameters
Simio was chosen to model the custom hip stem supply chain because it allows for easy updates and feature additions, the handling of large capacities relatively well, clear visualization of the evolution of the process, and experimental runs that simulate multiple scenarios simultaneously. As research continues, the Simio model will be adaptable and evaluation can be efficient.
The control variables used in this model are as follows: the number of medical engineers, the number of EBM operators/EBM machines, the number of CNC machinists/CNC machines, and the number of quality engineers. It was assumed that one EBM operator was capable of handling 3 machines and each CNC machinist needed a CNC machine. Therefore, the model uses a 3:1 ratio of EBM machines to operators and a 1:1 ratio for CNC machinists to machines.
The Simio model hypothesized that custom hip stem implants are a feasible new method for hip replacement. This simulation acknowledged that it is likely that not all patients will require customization due to the diminished difference between the estimated higher initial costs and expected custom-implant benefits for certain patients. Customization can be beneficial for unique cases, revisions, and younger patients. It is anticipated that 572,000 THAs will be performed in 2030; the revision rate and number of patients under 60 years of age are both increasing (Shan et al. 2014). The authors approximated an annual demand of 165,000 patient-specific hip stems because of the increasing eligible population. Patients arrive according to a Poisson arrival process meaning interarrival times are exponentially distributed to match the annual demand rate. One model entity was used to represent one patient order throughout the entire process. Patients that enter the system have already received a diagnosis requiring a custom hip implant.
The first server of the model was assumed to represent all medical facilities with CT scanning capabilities in the United States; in 2015 there were approximately 8,700 CT scanners in the United States (OECD 2018; United States Census Bureau 2018). The Simio model capacity for CT scanners allowed up to 8,700 scans to be performed at once. Although not all scanners would be available for THA patients simultaneously (nor are they in the same facility), CT machines were not considered a limiting factor for this model. The machines were assumed to operate 24 hours a day. For the CT scan, like most servers throughout this model, a triangular distribution was used to determine the processing time, because it has a definite lower bound, mean value, and upper bound. CT scanning time was given a triangular distribution (25, 30, 60) minutes as a conservative approach to account for the typical length of 15 to 30 minutes (including any scan preparation). The CT scans were assumed to be transported electronically to the medical engineer for CAD generation. Therefore, no time path was necessary.
The time that the medical engineer spends creating the CAD model of the patient-specific hip stem was given a triangular distribution (5, 8, 10) hours and the FEA duration a triangular distribution (3, 4, 5) hours. If the model fails FEA (a 5% chance), the time required to rework the CAD model and perform the subsequent FEA were both triangularly distributed with parameters (1, 4, 6) hours and (3, 4, 5) hours, respectively. The second FEA has a failure rate of 1%; if the implant does fail, the part was recirculated back into the original CAD model server. The medical engineers work 8-hour shifts 5 days a week. If a CAD model or FEA was incomplete at the end of a shift, the engineer suspended work until the next shift begins. These values were estimated by observing a small sample of these activities.
Time paths were included in the simulation to represent movement of the physical implant, but there was insufficient information for estimating the logistics (both between facilities and within the facilities themselves). Therefore, it was assumed that there was one processing facility where the fabrication, machining, testing, and packaging occur and the time for the implant to move throughout the facility was not considered because it did not affect resource time use. The EBM operator was responsible for batching the CAD model files and building any necessary support material for fabrication. It was assumed that the batch size would be the maximum capacity of the EBM machine: 14 implants (Cronskär et al. 2013). The maximum was used, because there are significant costs with each EBM batch printed that would be minimized by minimizing the total number of batches. Additionally, the throughput rate for the implants was expected to be high enough that waiting to batch the maximum number of implants would not add a substantial time onto the manufacturing time. The expected duration of batch file preparation was triangularly distributed (25, 30, 40) minutes. The print time for the EBM machine was 38 hours per batch. Each batch required an additional 10 hours in the EBM chamber to cool down (Cronskär et al. 2013). The machine preparation for batch printing was 65 minutes. The post-processing for the printed parts was 65 minutes for a batch size of 7 implants (Cronskär et al. 2013). Therefore, the post-processing time was extrapolated to 140 minutes for a batch of 14 implants. There was a 5% failure rate for builds and no failure/maintenance rate for the machines themselves. EBM operators finished work already started in batch file generation, preparation of the EBM machine or post-processing the parts before going off shift (build runs did not affect operators going off shift). Operators worked 8-hour shifts 5 days a week although the EBM machines ran nonstop
Each CNC machinist was responsible for the milling as well as manually grinding and polishing the implants. The implants were treated as individual pieces as opposed to batches in the finishing process. Each implant required 3 to 5 minutes uniformly to mill and the time to grind/polish was triangularly distributed (80, 90, 120) minutes. There was a zero failure/maintenance rate for CNC machines. The machinists worked 8-hour shifts 5 days a week; machinists finished work already started for each task before going off shift.
The quality engineer performed the QA inspection, washing, and packaging of the implant. A QA pass was implied in this supply chain model because the pass rate was expected to be high enough that neglecting failure would have insignificant impact on the overall system. The QA inspection duration was triangularly distributed (8, 10, 15) minutes. The implants were batched again to be washed and sterilized because these steps would likely be done in groups. The batch size was assumed to be 14 implants to hold consistent with the batching above. Ultrasonic washing of the implant was triangularly distributed (25, 30, 35) minutes. The time required to autoclave the batch of implants in sterile bags was uniformly distributed between 5 and 10 minutes. Package time for each individual implant (unbatched) was triangularly distributed (40, 50, 60) minutes. The quality engineers also worked 8-hour shifts 5 days a week. For each individual task, engineers finished work already started before going off shift.
singular location was intended to represent the average transportation across the United States. The majority of medical implants in the United States are shipped from Northeast Indiana. The average logistical load was modeled by the time to travel from this location to the population centroid of the United States (near Plato, Missouri), approximately 9.5 hours. The model did not use random generation for transportation times because this aspect of the model was not the focus of this research and, therefore, the authors did not want to complicate the results with widely varying transportation times. The model used a zero service time at the hospital server to represent no scheduling lapse between implant delivery and implantation surgery; although this was an unrealistic assumption, the hospital scheduling system was not pertinent to the focus of this research. The THA operation time was uniformly distributed between 1 and 2 hours. Similar to the CT scanning assumption above, the operations were assumed to be performed 24 hours a day, 7 days a week as it was not a constraining factor nor pertinent to this analysis.
Model Evaluation
To verify and validate the model, the input parameters were confirmed via the output values and “stress tests” were performed. For example, when certain resources were removed (e.g. Medical Engineers) no service occurred at those servers (CAD modeling, FEA) and the hip stems remained in the queue. The animation of the system in Simio allowed visual verification that the model ran smoothly from one server to the next. Each scenario had ten replicates to provide a sufficient random sampling of the model values and the scenarios were run at a 95% confidence interval. As parameters were estimated from existing research and data available, the authors conclude that the simulation model is valid. The determined control variable values represent the base case scenario for this research.
The model was evaluated on KPIs to determine system responses to control variable values. The KPIs consisted of average values for the system and were as follows:
- Production lead time – the time in the system or time from when the patient left the source (patient diagnosis) until the patient and hip entered the sink (after surgery),
- Modeling time – time required to create the CAD design file (including FEA and failures),
- Manufacturing time – time necessary to fabricate, finish, test, and package custom implants,
- Throughput rate – hourly rate custom hip stems move through the supply chain, and
- Resource utilization – the proportion of worktime the resource is working.
Resource utilization was considered a KPI for the control variables (number of medical engineers, EBM operators, CNC machinists, and quality engineers) and for the number of EBM machines. In addition to the KPIs, three other output parameters were identified. First, the resource utilization of the CT scan and surgery servers were examined to support assumptions made in the simulation implementation. The amount of powder used was also calculated. It was assumed that approximately 0.499 kilograms of Ti6Al4V powder were necessary to build one hip stem (Cronskär et al. 2013) and the EBM machine was 85% efficient with regards to powder use; industry is expected to increase efficiency from the research group’s study. This calculation disregarded ordering quantity of powder and recycling of excess powder.
Initially, the simulation system was empty; therefore, the system was given time to reach a steady state before the evaluation of KPIs. The system achieving steady state also demonstrates validation such that the number of parts entering the system was equivalent to the number of parts leaving the system. Steady state was determined by monitoring output values (e.g., average number of entities in the system, average time of entities in the system) until constant values were achieved.
termined. For one control variable at a time, a reduced and an increased value were each simulated to cause significant delay and allowance, respectively, on the overall system. There were 10 replicates of each scenario. The comparison was evaluated using a two sample t-test between the base case scenario and the high/low control variable scenario to determine a statistically significant difference in corresponding KPI values. An alpha value of 0.05 with a one-tailed p-value was used to determine significance. The goal of this model evaluation was to determine the sensitivity and general indications of the supply chain’s KPIs to particular resources to drive future work.
Results and discussion
Base Case Scenario
Initial control variable values were used from previous research by Shouche (2016). The preliminary results were analyzed, specifically the resource utilization, to establish variable values of the base case. Resources with very high utilization rates were increased to reduce the production lead time. Resources with low utilization rates were decreased, because a significant portion of that resource was not being used. Resource levels were changed one variable at a time and the simulation was run for an extended period of time and the output variables were monitored to ensure that the system reached steady state. Additionally, resource levels with high values were allowed for higher utilization rates. From the above systematic analysis of preliminary results from simulation trials, the model used 1,200 medical engineers, 35 EBM operators (105 EBM machines), 400 CNC machinists, and 125 quality engineers. The simulation required 30 simulated weeks to reach steady state. After 30 weeks simulation time, 94,886 custom hip stems were implanted into patients, completing the supply chain flow. Extrapolating to a full year, 165,034 custom hip stems would be implanted using approximately 60,000 kilograms of Ti6Al4V powder. The KPIs and other output variables are summarized in Table 1.
Table 1: KPIs and additional output variables for the base case control variable values.
| KPIs | Value | Additional Output Variables | Value |
|---|---|---|---|
| Production Lead Time | 170.5 hrs | Average Number in System | 3277.7 |
| Manufacturing Time | 99.6 hrs | Ti6Al4V Powder Used | 59,861.0 kg |
| Modeling Time | 58.6 hrs | Maximum CT servers used | 31.4 |
| Throughput Rate | 18.8 hr-1 | CT server utilization | 14.4% |
| Medical Engineer utilization | 83.6% | Maximum surgery servers used | 237.0 |
| EBM Operator utilization | 51.1% | Surgery utilization | 28.3% |
| EBM Machine utilization | 66.1% | ||
| CNC Machinist utilization | 65.9% | ||
| Quality Engineer utilization | 31.8% |
The availability and capacity assumptions for the CT scan and surgery servers were confirmed by the simulation. The amount of time and resources required to complete each was trivial: neither server was a bottleneck as demonstrated by a low average maximum units utilized (31.4 for CT scanners and 237 for physicians in surgery) within the system. The number of CT machines and capacity for surgery can widely vary without changing the system’s output significantly. Additionally, the throughput rate of the overall system was nearly 19 implants per hour indicating that batching 14 implants for EBM printing and sterilization would add less than an hour on average to the production lead time per task.
The total number of medical engineers in the system was high; additionally, the medical engineer utilization was also very high. Due to the service time of this server (an average time of 58.6 hours), the capacity must be very large in order to avoid a large backlog. The EBM machines had a higher utilization rate than the EBM operators which could indicate that the number of EBM machines drive the requirement for that resource rather than the operator labor. The quality engineers had a low utilization rate, as shown in Table 1, but small decreases in the resource level led to significant changes in the utilization rate.
The throughput rate for patient-specific hip stems was 18.8 implants per hour. The average total time the patients/hip stem implants were in the system was 170.5 hours (approximately one week). The processing time for the base case scenario of a custom hip stem prosthesis is expected to be sufficient for scheduling of elective THA surgeries.
Sensitivity Analysis
From the base case scenario, viable ranges were determined for each control variable to stress and relieve the system. The low and high values were [800, 1,600] for medical engineers, [20, 50] for EBM operators, [200, 600] for CNC machinists, and [75, 175] for quality engineers. A two sample t-test between the base case scenario and the high/low scenario were calculated and the statistical significance of the populations were determined. An alpha value of 0.05 and the one-tailed p-value was used. Table 2 displays those scenarios that had a statistically significant difference from the base case scenario with an ‘X’. Those populations in which the null hypothesis could not be rejected are shown with a ‘-’. The purpose of this analysis was to provide general indications of control variable effects on the KPIs to support future work.
Table 2: The table shows which cases of varied control variables had a statistically significant difference from the base case (X). Those cases that were not significantly different are marked with a dash (-).
| KPI | Medical Engineers | EBM Operators | CNC Machinists | Quality Engineers | ||||
|---|---|---|---|---|---|---|---|---|
| High | Low | High | Low | High | Low | High | Low | |
| Production Lead Time | - | X | X | X | - | X | X | X |
| Manufacturing Time | X | X | X | X | - | X | X | X |
| Modeling Time | X | X | - | - | - | - | - | - |
| Throughput Rate | - | X | X | X | - | - | X | X |
| Medical Engineer utilization | X | X | - | - | - | - | - | - |
| EBM Operator utilization | X | X | X | X | - | - | - | - |
| CNC Machinist utilization | X | X | X | X | X | X | - | - |
| Quality Engineer utilization | - | X | X | X | - | X | X | X |
There are two major conclusions drawn from Table 2. First is that, intuitively, strain in the beginning of the supply chain causes rippling effects to the following servers. A low level of medical engineers caused a significant effect on all KPIs; each KPI was a measure of the supply chain that either included or followed the modeling stage. Similarly, a low level of EBM operators (and therefore EBM machines) had a significant effect on all following KPIs (the modeling time and utilization of medical engineers were measures of the supply chain prior to fabrication). For production lead time and the throughput rate, a high level of medical engineers did not have a significant effect. This could indicate that though it improved the modeling time, the supply chain had other limiting factors that caused equivalent total processing time and throughput rate to the base case. For example, the utilization of EBM operators was relatively high in the base case scenario and as there was a positive t-value associated with a high level of medical engineers, the number of EBM operators and machines could be a contributing factor to the lack of improvement.
The second conclusion is that variables in this simulation were likely to have threshold values, above which increasing the control variable does not improve supply chain performance significantly. An alternative explanation for the lack of significant effect on particular KPIs by the increased level of medical engineers could be that the threshold value was surpassed. The significant difference between the high level of EBM operators and every KPI (including or following fabrication in the supply chain) could indicate that the threshold was not achieved for this control variable.
Another observation is that particular variables could vary significantly relative to their base case value. For example, CNC machinists varied by 50% and though utilization had convincing effects, the throughput rate was unaffected. In contrast, the number of medical engineers was increased and decreased by a third of the base case value and this led to strong KPI effects, shown in Table 2.
Summary and future work
The anticipated AM supply chain of custom hip stems was developed and simulated in this study. The custom hip stem is a good candidate for EBM fabrication because a significant portion of the prosthesis surface can be finished by the EBM machine; in other words, there is a significant portion of rough surface on implant for osseointegration that does not require machine finishing. This aspect makes hip stems a particularly good candidate for customization using EBM manufacturing. The supply chain begins when a patient is in need of a THA and is a good candidate for a custom implant. The patient must have a CT scan to begin the implant CAD modeling process. A medical engineer designs a CAD model of the implant and performs FEA to ensure the model will not fail. Then the CAD file is transferred to an EBM operator who batches several files into a build file and prints the file on an EBM machine. There is some post processing required by the EBM operator and the hip stems are individually dispatched to CNC machinists to be milled, grinded, and polished. A quality engineer does final quality assurance testing, sterilization, and packaging of the prosthesis for transportation. The package is individually transported to the hospital where it is held until the THA operation where the hip stem is implanted in the patient. Parameters were determined from previous studies and the supply chain was simulated in Simio.
Using information from the base case scenario and the high/low analysis, the number of medical engineers is critical to the success of the supply chain. First, there is a relatively high number of medical engineers required for the supply chain to run without significant delay. Second, delays at the modeling step of the process related to significant effects on the rest of the supply chain. EBM machines seem to drive effects seen by the number of EBM operators; this is likely due to the lengthy processing time of the machine compared to the total processing time by the operators. The number of CNC machinists could vary significantly and though the resource utilization increased drastically, the throughput remained unaffected. The number of quality engineers only affected the end of the supply chain. Although each control variable was analyzed separately, it is likely that there are interactions among the variables that affect the KPIs.
This model is novel from previous studies, particularly supply chain research by Shouche (2016), because it provides a more detailed, robust analysis of the custom supply chain. A unique feature of this analysis is that every customer who enters the system, successfully leaves the system with a customized implant. Additionally, studies have been conducted that provide parameter estimation for specific steps of the supply chain instead of estimating general times. Broad processes were broken down into more detailed steps to better analyze resource use. Overall, this simulation focuses significantly more on the resource requirement and utilization of the supply chain. This study also found a production lead time for patientspecific hip stems. Further, the sensitivity analysis in this study observes the statistical significance of resource level variation on various KPIs which is a substantial advancement to the previous study. Though both studies agreed on the extreme sensitivity of medical engineers, this study found important differences with other resource levels, such as quality engineers, that were previously undiscovered.
With regard to this research, exploration of resource boundaries is necessary. Further, a regression analysis could better determine relationships between varying control variables and output variables/KPIs. The results demonstrated by Table 2 indicate that there are likely main effects and interactions among the control variables on the output variables. Determining these relationships may provide more insights into supply chain requirements.
pply chain requirements. Future work would also consider the financials of the system. Significant market insights were foregone in this analysis because they depend largely on the economics of the supply chain. The feasibility of the system is partially dependent on the economic feasibility of patient-specific hip stems. The initial cost as well as the annual operating cost to the producer are a major factor on the capital investment decision to enter into AM custom implants. Additionally, the cost of the custom prosthesis influences the patient (and more likely insurance companies) on whether the implant is a viable option. The benefits for particular patients must outweigh the expected increased cost of the implant. In the financial analysis, a comparison to traditionally manufactured, made-to-stock implants would be beneficial as there is a significant inventory cost associated with made-to-stock implants that could be eliminated with customized prosthesis.
Another avenue of future work includes an analysis where stock-size parts and other types of implants are also additively manufactured. AM allows for flexibility in build files which could support both custom and stock implants, of multiple types, to be manufactured together. The supply chain would expand and the resource levels would change disproportionately (e.g., CAD modeling would not be necessary for standard implants, more machining may be required for other types of implants).
plants, more machining may be required for other types of implants). Overall, the development of the anticipated supply chain and implementation of the simulation provides an advancement on previous research and a sound starting point for future detailed analysis into the feasibility and application of AM patient-specific medical devices.
Acknowledgements
The author gratefully acknowledges Dr. Robert Golz, MD for the invaluable information about general medical practices and orthopedics and Dr. Ola Harrysson for his wealth of knowledge on additive manufacturing and biomedical engineering.
References
Arcam AB, 2018. EBM Inside: Additive Manufacturing for Orthopedic Implants . http://www.arcam.com/wp-content/uploads/EBM-Inside.pdf, accessed 27.02.2018.
Arcam EBM, 2018. Arcam History. http://www.arcam.com/company/about-arcam/history/, accessed 27.02.2018.
Cronskär, M., M. Bäckström, and L. Rännar. 2013. “Production of Customized Hip Stem Prostheses – a Comparison Between Conventional Machining and Electron Beam Melting (EBM)”. Rapid Prototyping Journal 19(5):365-372.
Harrysson, O. L. A., Y. A. Hosni, and J. F. Nayfeh. 2007. “Custom-designed Orthopedic Implants Evaluated Using Finite Element Analysis of Patient-specific Computed Tomography Data: Femoral Component Case Study”. BMS Musculoskeletal Disorders 2007 8:91.
Harrysson, O. L. A., D. R. Cormier, D. J. Marcellin-Little, and K. R. Jajal. 2003. “Direct Fabrication of Metal Orthopedic Implants Using Electron Beam Melting Technology”. In Proceedings of Solid Freeform Fabrication Symposium, August 4th-6th, Austin, Texas, 439-446.
Haslauer, C. M., J. C. Springer, O. L. A. Harrysson, E. G. Loboa, N. A. Monteiro-Riviere, and D. J. Marcellin-Little. 2010. “In Vitro Biocompatibility of Titanium Alloy Discs Made Using Direct Metal Fabrication”. ScienceDirect: Medical Engineering & Physics 32:645-652.
OECD Data, 2018. Computer Tomography (CT) Scanners. https://data.oecd.org/healtheqt/computedtomography-ct-scanners.htm, accessed 28.02.2018.
OrthoInfo, 2017. Treatment: Total Hip Replacement. http://orthoinfo.aaos.org/topic.cfm?topic=a00377, accessed 27.11.2017
Shan, L., B. Shan, D. Graham, and A. Saxena. 2014. “Total Hip Replacement: A Systematic Review and Meta-Analysis on Mid-term Quality of Life.” Osteoarthritis and Cartilage 22:389-406
Shouche, S. 2016. Supply Chain Operations Reference Model for U.S. Based Powder Bed Metal Additive Manufacturing Process. Master's Thesis, Department of Industrial Engineering, North Carolina State University, Raleigh, North Carolina. https://repository.lib.ncsu.edu/handle/1840.16/11030.
Sing, S. L., J. An, W. Y. Yeong, and F. E. Wiria. 2015. “Laser and Electron-Beam Powder-Bed Additive Manufacturing of Metallic Implants: A Review on Processes, Materials and Designs”. Journal of Orthopedic Research 34:369-385.
Thomsen, P., J. Malmström, L. Emanuelsson, M. René, and A. Snis. 2008. “Electron Beam-Melted, FreeForm-Fabricated Titanium Alloy Implants: Material Surface Characterization and Early Bone Response in Rabbits”. Journal of Biomedical Materials Research 90B(1):35-44.
United States Census Bureau, 2018. U.S. and World Population Clock. https://www.census.gov/popclock/, accessed 28.02.2018.
Zhang, L. C., E. C. S. Kiat, and A. Pramanik. 2009. “A Briefing on the Manufacture of Hip Joint Prostheses”. Advanced Materials Research 76-78:212-216.
Author biographies
MARGARET GOLZ is currently a PhD candidate in Operations Research at North Carolina State University. She holds a BS in Chemical Engineering and an MS in Engineering and Technology Management from Colorado School of Mines. Her e-mail address is mgolz@ncsu.edu.
RICHARD WYSK is the Dopaco Distinguished Professor of Industrial and Systems Engineering in the Edward P. Fitts Department of Industrial and Systems Engineering at North Carolina State University. Prior to joining NC State, he held chaired positions at Texas A & M University and Penn State University. He has written or contributed to more than a dozen books focused on product and process engineering and is the author or co-author of more than 175 journal papers. A Fellow of the Institute of Industrial Engineers (IIE) and the Society of Manufacturing Engineers (SME), he is also the recipient of the IIE David Baker Outstanding Research Award and the IIE Albert Holzman Distinguished Educator Award. His email address is rawysk@ncsu.edu.
RUSSELL KING is the Henry A. Foscue Distinguished Undergraduate and Graduate Professor of Industrial and Systems Engineering at North Carolina State University in Raleigh and is a Fellow of the Institute for Industrial and Systems Engineers (IISE). At NC State, he is Co-Director of the Center for Additive Manufacturing and Logistics (camal.ncsu.edu). He has consulted for a number of companies including Ford Motor Company, The Gap, Dillards Department Stores, and the Institute for Defense and Business. He has received number of awards for his teaching, advising and research including the Albert G. Holzman Distinguished Educator Award and the Technical Innovation in Industrial Engineering Award from IISE. In his spare time he is a Master (4th degree black belt) of Taekwon Do studying under Grand Master K.S. Lee in Morrisville, NC, USA. His email address is rawysk@ncsu.edu.
COLIN NOLAN-CHERRY is currently employed at Citrix Systems, Inc. He holds a BS in Industrial and Systems Engineering from North Carolina State University. His email address is cdnolanc@ncsu.edu.
STEPHEN BRYANT is currently a student at North Carolina State University. He intends to graduate in Spring 2019 with a BS in Industrial and Systems Engineering. His email address is sjbryan4@ncsu.edu.
